An acute disease of young geese described since the 1970s in France, Hungary and Germany, hemorrhagic nephritis enteritis (HNEG) is a contagious viral disease responsible for high mortality in breeding or force-feeding. It is still a major constraint on goose production, particularly in southwestern France. The etiology of the latter was determined 30 years after the first case by Guerin et al.
The disease agent and its pathogenicity
The HNEG agent is a virus of the genus Polyomavirus, goose polyomavirus (GHPV or Goose Hemorrhagic Polyomavirus), of the family Polyomaviridae. The virions, not wrapped, are icosahedral in shape, 40 to 50 nm in diameter. The capsid is composed of 72 pentameric capsomers.
The GHPV genome consists of a double circular DNA strand of 5256 bp, divided into 2 main regions : one region coding for regulatory proteins, and the other for 3 structural proteins.
This virus is very resistant, especially to heat (still virulent after 2 hours of exposure to 55°C), cold, lipid solvents.
GHPV is detected in geese but also in ducks, the latter acting as a reservoir of the pathogen, being also a healthy carrier.
Epidemiological data
The first description of HNEG dates back to 1969 in Hungary. During the 1970s, the disease was observed in Germany and France. Currently in France, the disease is still observed episodically in the southwest. It is also reported in the Western region.
Geese and ducks (Muscovy and Mulard ducks) are susceptible to infection with GHPV at any age. Infection of other anatids with GHPV has not yet been demonstrated. On the other hand, only the goose is susceptible, as the duck shows no clinical signs or lesions following infection.
Experimentally, the goose’s sensitivity window typically extends between 3 and 12 weeks. Sometimes clinical forms may appear later.
The importance of co-factors in triggering the clinic is demonstrated. Overfeeding at the beginning of breeding, poor housing conditions, stress (handling, vaccination, climate with increased prevalence in winter…) are factors to look for in case of clinical expression of HNEG.
The transmission routes are still not well known. Horizontal transmission is demonstrated by the fecal-oral route. Vertical transmission of GHPV cannot be excluded but is still not demonstrated.
Healthy carrying of the virus is common in geese, especially adults, with long persistence in infected geese.
Duck is considered a potential reservoir of GHPV. The prevalence of GHPV infection (and transmission) in duck farming is still unknown but appears high. Cohabitation between infected geese and ducks can therefore introduce the virus into geese, without any clinical manifestation in ducks.
Clinical manifestations of the disease
In geese aged 4 to 10 weeks, morbidity can reach 80% and lethality is variable but can approach 100%. The older the geese are, the lower these values are.
Symptoms
HNEG incubation lasts one to two weeks depending on the infectious load, susceptibility of the geese and the age of the individual. The clinical period is generally short (1 to 3 days). Geese isolate themselves in a comatose state, are amorphous. They have difficulty moving around and stop consuming water and food. Discreet diarrhoea is common. Nervous signs such as pedaling, opisthotonos are observed in the acute forms in young animals. Subjects showing clinical signs die within a few hours.


Lesions
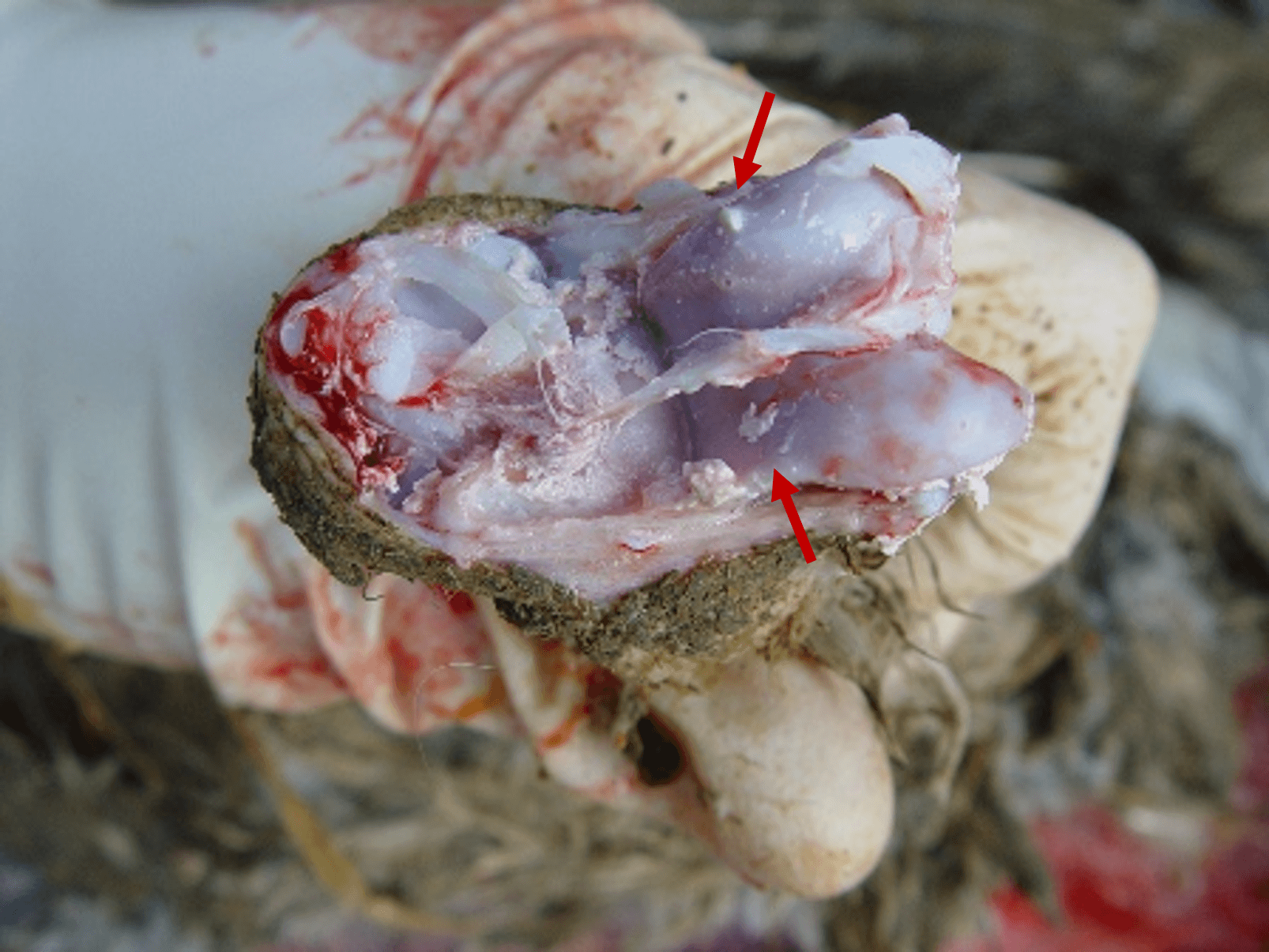
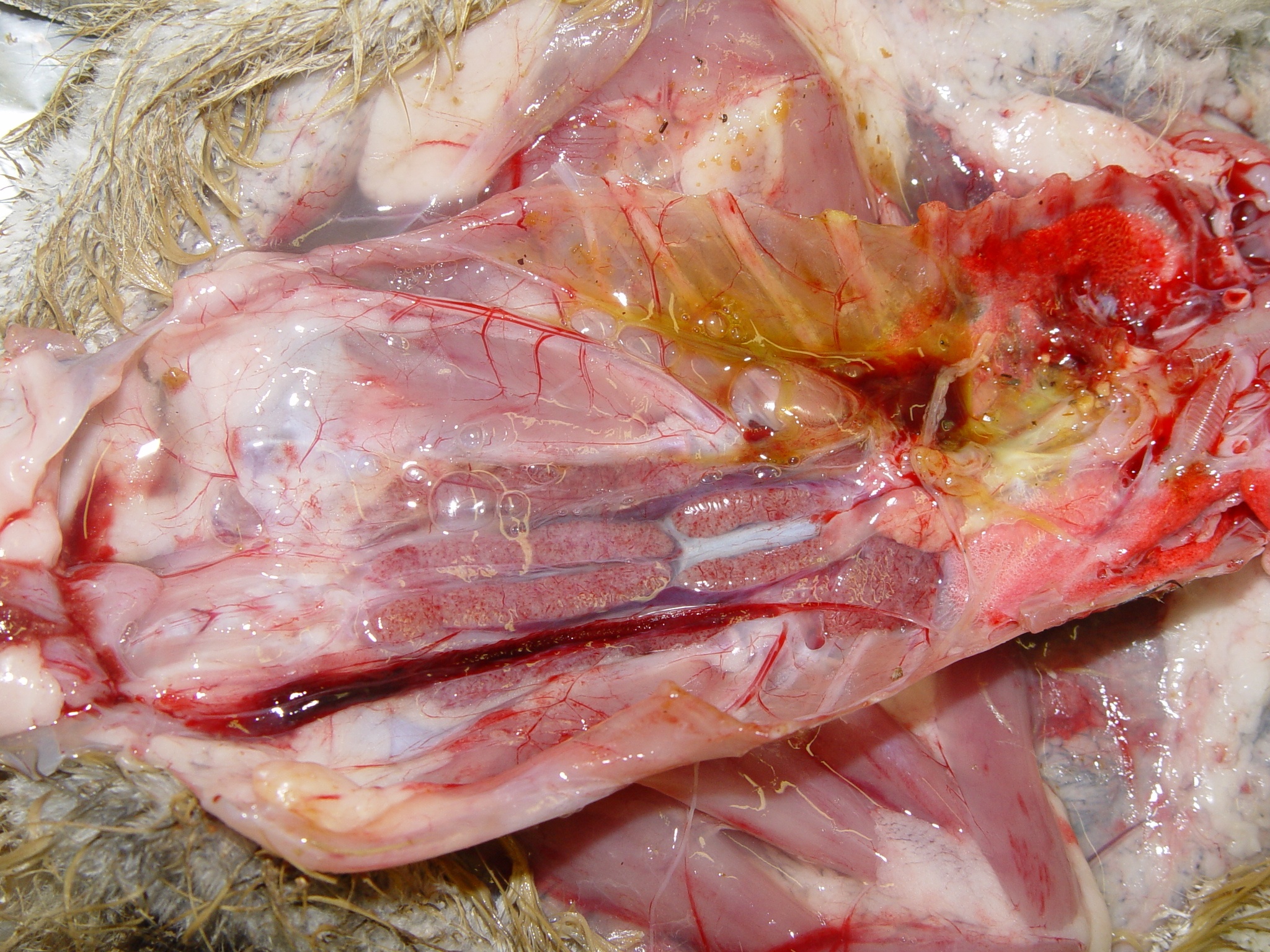
The autopsy lesions are quite characteristic. Yellowish gelatinous ascites and subcutaneous gelatinous edema are frequently observed. The kidneys often have hemorrhagic nephritis or urate overload (puffy and white appearance), due to the subject’s dehydration. Muco-hemorrhagic enteritis is also observed, with the presence of partially digested blood.
In late forms (older animals, lower infectious dose), visceral drop lesions, with urate deposits on the organs of the thoracoabdominal cavity, are often observed. These deposits can also be observed in the joints. The spleen may be enlarged and hemorrhagic. Sometimes, hepatitis is observed with a dark red enlarged liver and lighter areas.
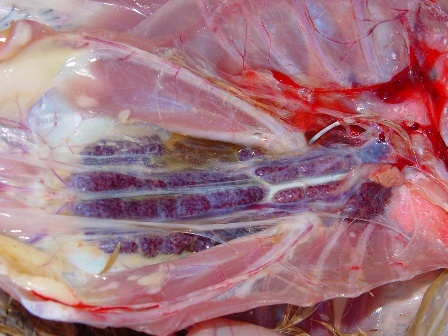
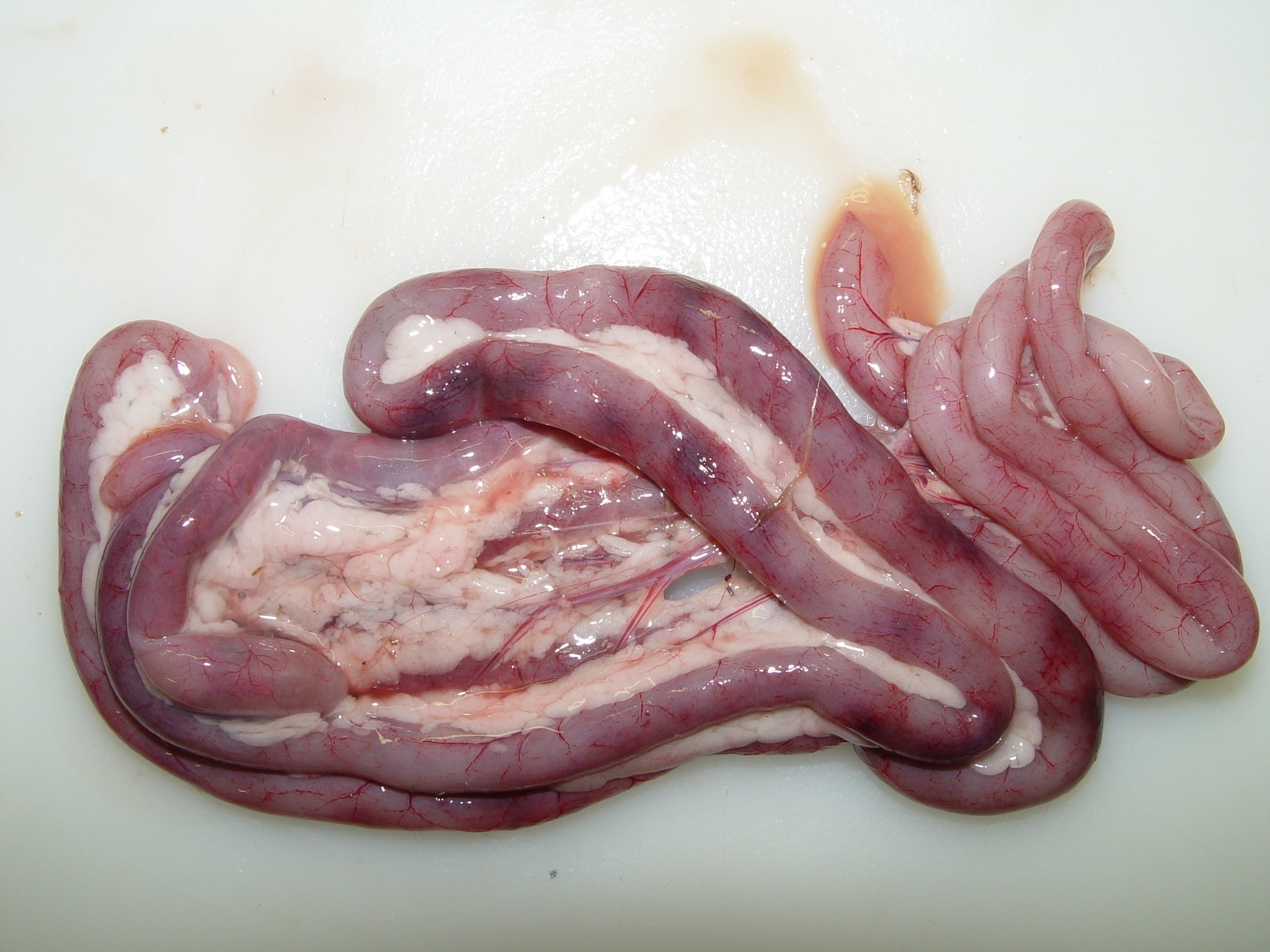
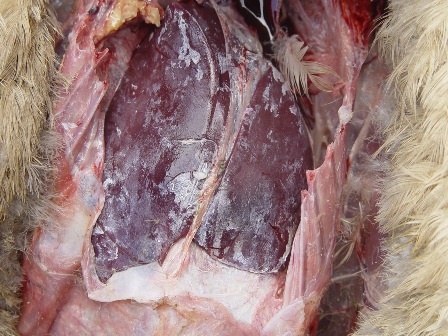
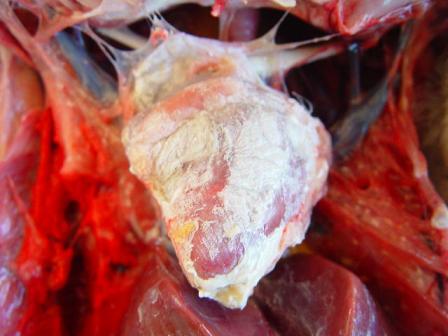
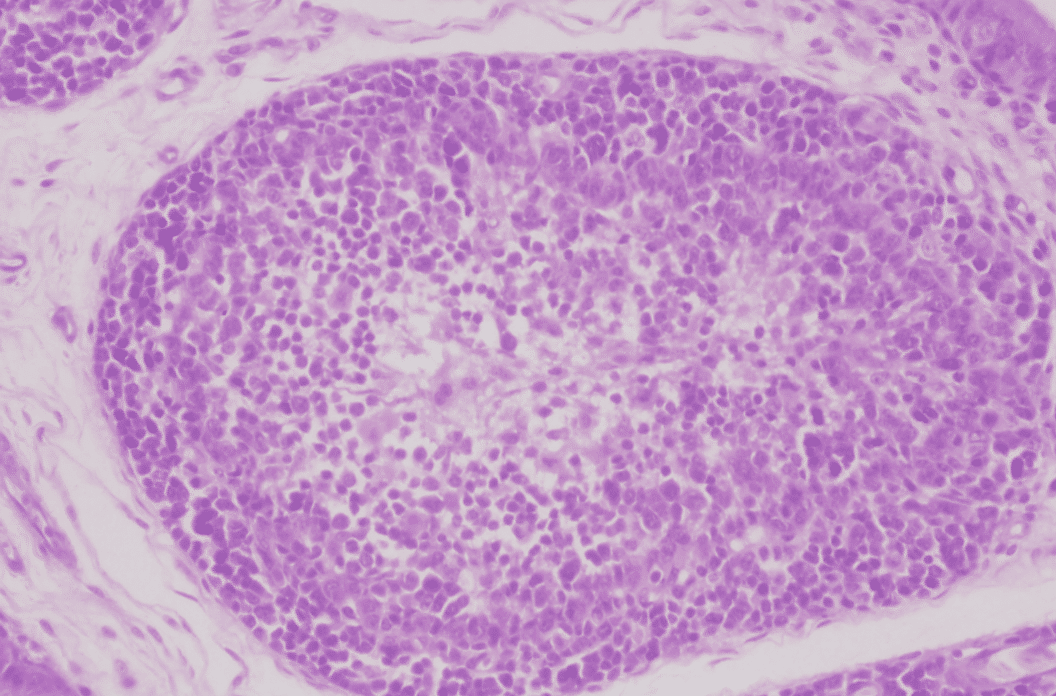
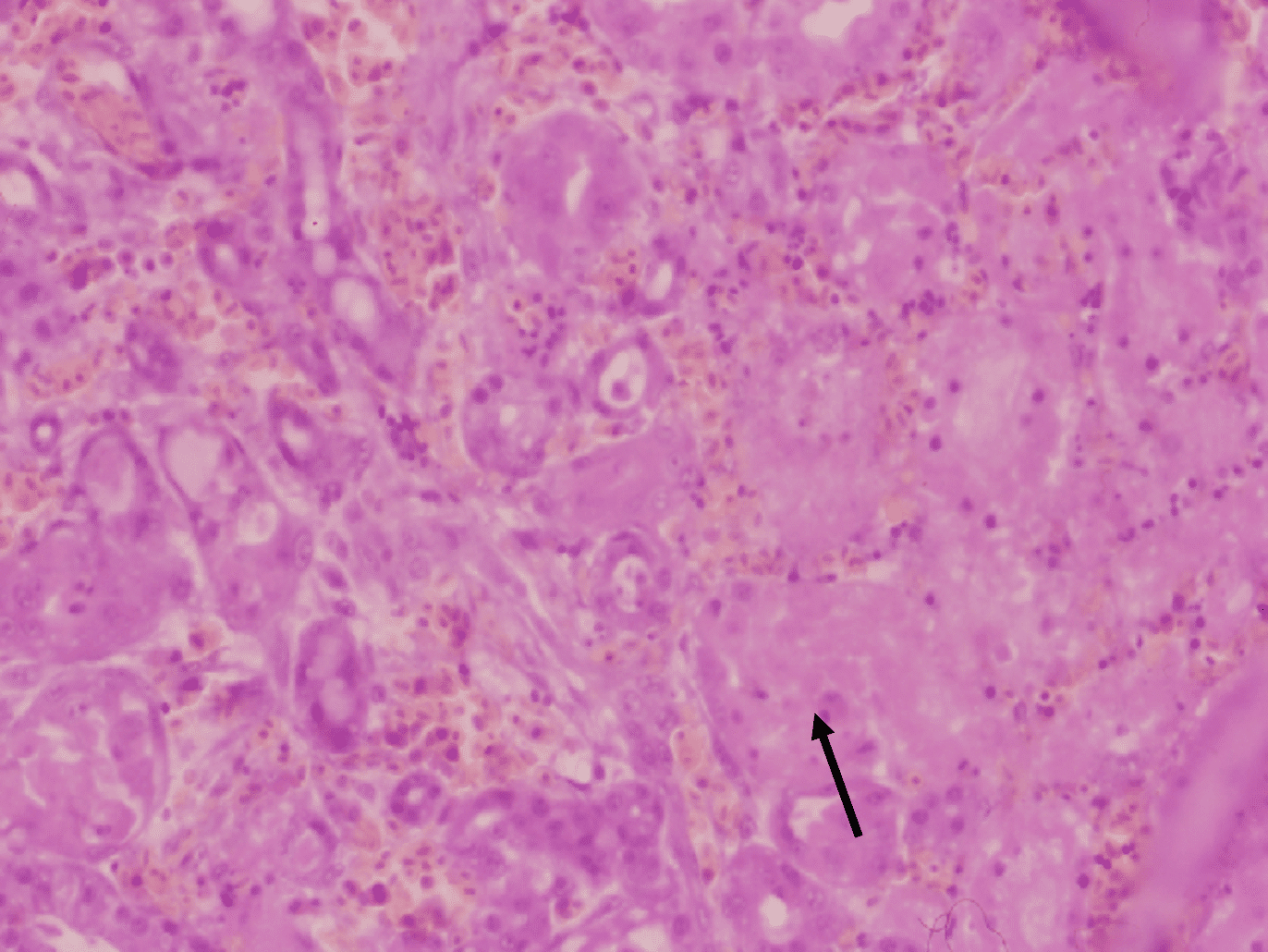
Histopathological examination shows that GHPV replicates in almost all tissues, with a preferential tropism for endothelial cells and lymphoid cells (particularly B lymphocytes). The most notable lesions are necrosis of the tubular epithelium of the kidneys, lymphoid depletion of the Fabricius bursa and spleen, necrotico-hemorrhagic foci of the digestive tract (duodenum and jejunum).
The immunity developed in geese following infection is poorly known. Neutralizing antibodies are detected in naturally or experimentally infected geese. Their transmission to offspring (maternal antibodies) appears to be effective. The duration of immunity acquired following infection or through the transmission of maternal antibodies would be 3 weeks.
The diagnosis
Epidemio-clinical suspicion
Based on a high mortality of young geese in breeding, associated with classic lesions of nephritis and haemorrhagic enteritis, ascites, sometimes visceral or joint gout.
Confirmation of the diagnosis in the laboratory
- Histology : necrosis of the intestinal mucosa, inflammation of the renal interstitium, follicular lympholysis in the Fabricius bursa.
- Virology : a diagnostic technique for GHPV infection by PCR is routinely available in France and allows detection of viral DNA in the spleen, liver, kidney, in a few days.
Differential diagnosis
Derzsy’s disease / Avian cholera / Herpesvirus
Disease prevention and control
Prevention
There is no effective treatment. In an infected lot, geese expressing clinical signs die within a few hours. Geese without clinical signs must be bred under husbandry conditions that minimise the risk of stress factors. It may therefore be justified to reassess the appropriateness of vaccinations on these animals (especially pasteurellosis vaccination, which presents risks of secondary reactions).
Given the high resistance of the virus, at the end of the breeding of a sick batch, a strict and rigorous cleaning and disinfection protocol must be applied (use of chlorinated derivatives). A sufficiently long crawl space and a rotation of the outside areas must be observed.
Prevention is based on hygiene and compliance with strict health protection measures. Single-band driving is preferable.
Vaccination
A vaccine has been developed on an experimental basis at the ENVT (Ecole Nationale Vétérinaire de Toulouse). In a high-risk batch, vaccination of growing geese can induce active immunity. Vaccination of breeding animals is recommended to protect the offspring, by 2 administrations, before each laying period.
- GUERIN J.L., GELFI J., DUBOIS L., VUILLAUME A., BOUCRAUT-BARALON C., PINGRET J.L. A novel polyomavirus (goose hemorrhagic polyomavirus) is the agent of hemorrhagic nephritis enteritis of geese. Journal of Virology, 2000, 74: 4523-9.
- LACROUX C., ANDREOLETTI O., PAYRE B., PINGRET J.L., DISSAIS A., GUERIN J.L. Pathology of spontaneous and experimental infections by Goose haemorrhagic polyomavirus. Avian Pathology, 2004, 33: 351-8.
- PINGRET J.L., BOUCRAUT-BARALON C., GUÉRIN J.L. Goose haemorrhagic polyomavirus infection in ducks. The Veterinary Record, 2008, 162: 164.
- GELFI, J., PAPPALARDO, M., CLAVERYS, C., PERALTA, B. & GUERIN, J-L. (2010). Safety and efficacy of an inactivated Carbopol-adjuvanted goose haemorrhagic polyomavirus vaccine for domestic geese. Avian Pathology, 39(2):111-6.







