Gumboro disease, first described in 1962 in the city of Gumboro (Delaware, USA), is probably one of the most important diseases in the world today due to its economic importance.
Severe forms of Gumboro disease appeared in Europe in 1987, associated with “hypervirulent viruses”. These highly pathogenic strains then spread to many countries.
The disease agent and its pathogenicity
The causal agent is a birnavirus (Infectious bursal disease virus = IBDV, Avibirnavirus specific to poultry) : this virus is non-enveloped and its genome consists of two segments of double-stranded RNA, hence the name “bi-RNA”.
A birnavirus (not classified as Avibirnavirus) also causes Proventricular necrosis in chickens. Other birnaviruses affect fish, molluscs and insects.
Concerning IBDV, two serotypes exist : serotype I is the only pathogen for chicken and 6 distinct strains have been identified.
There is no cross protection : anti-serotype II antibodies do not protect against serotype I.
A very virulent form of the virus (vvIBDV for very virulent IBDV), close to serotype I, is emerging.
The virus has no zoonotic potential.
Chicken is the natural host of the virus. Birds are more sensitive between 3 and 6 weeks of age.
Chicks infected before the age of 3 weeks develop immunosuppression which can lead to severe economic losses.
This virus attacks immature B lymphocytes and causes lympholysis in the Fabricius bursa. Other lymphoid organs, such as the thymus, spleen and caecal tonsils, are also affected. The disease can thus severely compromise the humoral immunity of affected chicks when they are less than 3 weeks old at the time of infection. Maternal immunity is therefore very important for the protection of these young birds.
Epidemiological data
The virus is transmitted horizontally, directly and indirectly.
The disease is highly contagious and the incubation period is short, 2 to 3 days. There is no vertical transmission.
This virus is highly resistant to most disinfectants (iodine derivatives, phenols, quaternary ammoniums, cresols…) and in the environment, surviving for months in poultry houses and for weeks in food, water and droppings.
Studies have also highlighted the role of the litter beetles (small beetles) in the transmission of the virus.
Clinical manifestations of the disease
There are classically 3 expressions of the disease :
- The immunosuppressive form
It concerns chicks less than 3 weeks old, with little or no protection from maternal antibodies. This form of infection does not result in acute mortality, but is often associated with superinfections that are often devastating (gangrenous dermatitis ; colibacillosis, hepatitis with inclusions, etc.).
This form is almost non-existent in industrialized countries, due to the systematic vaccination of breeding animals. - The clinical form
The clinical form is observed after 3 weeks of age (mainly between 3 and 6 weeks of age when the Fabricius bursa is functional).
Morbidity is very high (50 to 100%) and mortality is around 20-30% for classical strains (but can be around 100% for very virulent strains).
The episode is often very brief (4 to 7 days). Sick birds show despondency, anorexia, feather ruffling with diarrhea and dehydration.
Due to vaccination, clinical forms are mainly due to variant forms and sometimes very virulent (vvIBDV). - The subclinical form
Early infection leads to immunosuppression, without the characteristic signs of the clinical form, followed later by various secondary infections. At necropsy, these birds will also show a marked change in the bursa, in addition to other lesions related to secondary infection.
Lesions
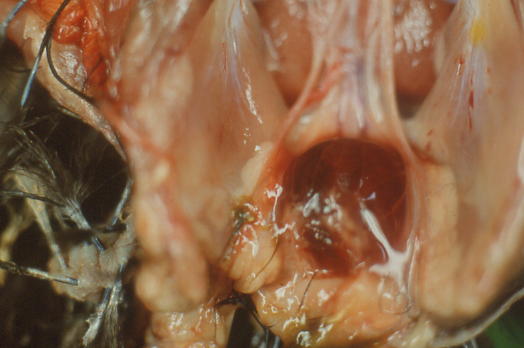
Dehydration, intramuscular haemorrhages are observed on the carcass with, at the beginning of the infection, an edema of the Fabricius bursa sometimes accompanied by bleeding. Bleeding is probably related to the formation of complex immunities.
This swelling will be followed, 7 days after infection, by severe atrophy of the bursa. The bursa will therefore be of increased size in the first days after infection (due to oedema) and then gradually reduce in size.
At histology, there is a necrosis of affected lymphocytes in different lymphoid organs, the bursa being by far the most affected. The follicles of the Fabricius bursa therefore show lymphoid depletion with lymphocyte destruction and subsequent atrophy, accompanied by an influx of heterophilic polynuclear cells (equivalent to mammalian neutrophils). Similar changes will also be present in other lymphoid organs (spleen, thymus, caecal tonsils…) and will often be more marked for very virulent strains.

The diagnosis
Epidemio-clinical diagnosis
First diagnosis : acute mortality (over a period of less than 5 days) and damage to the Fabricius bursa ; it is easy in the case of acute clinical episodes.
Differential diagnosis
Infectious anemia, Malabsorption syndrome, coccidiosis, …
Experimental diagnosis
Histological examination of the Fabricius bursa is valuable, especially at early stages of infection : the morphology of the Fabricius bursa can vary considerably depending on the stage of disease progression, so several animals should be analysed.
Isolation and identification of the virus
It is rarely implemented because it is too expensive ! In a research context, the use of monoclonal antibodies or sequence analysis can be used to characterize an isolate and in particular to identify a possible variant.
Antigen rapid IBDV detection kits, implemented on Fabricius bursa tissue fragments, are now available in the field.
Serology (ELISA)
Only kinetics (2 samples at 3-week intervals) can be interpreted, in particular to monitor the vaccination response in breeding and growing chickens.
ELISA does not distinguish between serotypes I and II.
It should also be noted that new generations of ELISAs could make it possible to distinguish between infected and vaccinated animals (DIVA strategy, Differentiate Infected from Vaccinated Animals).
Disease prevention and control
Compliance with biosecurity rules is essential to limit the risk : it is important to recall the importance of the crawl space and compliance with the cleaning-disinfection protocol.
However, given the omnipresence of the virus, vaccination prevention is essential and widespread, particularly among breeding animals. As we have seen, the presence of neutralizing maternal antibodies is crucial to prevent early replication of the virus.
Vaccination against Gumboro disease is therefore based on 2 complementary approaches:
- Vaccination of breeding animals to transmit maternal antibodies to the chick : this is done with an inactivated and adjuvanted booster vaccine before laying.
- Vaccination of growing chicks, to relay this passive protection : it is done with live attenuated vaccine :
This vaccination must be adapted to the level of maternal antibodies and the risk of contamination (high infection pressure ? risk of highly pathogenic strain ?)
To adapt the vaccination of growing chicken, 2 parameters are essential :
- The age of vaccination of the chick : it is necessary to vaccinate early enough not to leave the chick without antibodies, but late enough to avoid neutralization of the vaccine by the maternal antibodies. This adjustment requires the determination of the maternal antibodies level at 1 day and the modelling of the decrease in serum antibodies. A mathematical model – the Kouwenhoven formula, of which there are variants – makes it possible to determine the optimum age of vaccination as a function of the ELISA titer at 1 day. This decrease is influenced by the growth rate, which is a dilution factor for maternal antibodies ! (fast-growing chickens will see their ELISA titer decrease faster than future slow-growing laying chickens)
- The vaccine strain, more or less attenuated : there are highly attenuated vaccine strains, called “light”, strains with “intermediate”, “intermediate plus” pathogenicity and strains with high residual pathogenicity, called “hot” strains : these “hot” ones are of very limited use in the field given the danger of their use.
- New vaccine technologies are now applicable to the hatchery : recombinant HVT-IBDV vaccine (Vaxxitek©, Merial) or virus-Antibodies immuncomplexes.
These two approaches have in common that they are free from neutralization by maternal antibodies. They are also applicable by in ovo vaccination, to the transfer of hatching eggs (18-19 days of incubation).







